Susan Hepworth, Bob Hopkins, Jr., MD, Jefferson Jones, MD, Karen Crowley, DNP

Susan Hepworth: Thanks, everybody, for joining. We’ve got almost everyone who RSVP’d has joined. We will get started with today’s webinar, Protecting Infants from RSV: Understanding Guidance on New Prevention Tools. My name is Susan Hepworth. I serve as executive director at the National Coalition for Infant Health, one of the hosts of today’s webinar. I am delighted to be joined by three speakers here today with us.
We’re joined by Dr. Kieran Crowley of the Association of Women’s Health, Obstetric and Neonatal Nurses. We’re also joined by Dr. Jefferson Jones of the CDC and Dr. Bob Hopkins of the National Foundation for Infectious Diseases.
I want to recognize the co-hosts of today’s webinar, the Association of Women’s Health, Obstetric and Neonatal Nurses and NFIB, the National Foundation for Infectious Diseases. I also want to thank our sponsors, Merck, Pfizer, and Sanofi, who helped make today’s webinar possible. To quickly outline the objectives of today’s webinar, we will receive an overview of RSV from Dr. Bob Hopkins at NFID. Then, we will hear from Dr. Jefferson Jones about new options to prevent RSV and what the CDC guidance says about their use. Then, we will hear from Dr. Karen Crowley, who wants to talk about resources for providers, patients, and caregivers to educate about these new prevention tools.
We have reserved a few minutes at the end for Question and Answer, so feel free to send those questions as they come to your mind. With that, I will start with a concise video that the National Coalition for Infant Health produced last year, based on a surveyconducted at the end of 2022.
Video https://www.infanthealth.org/rsv#videos
Nearly every child catches RSV by age two. Respiratory Syncytial Virus affects the lungs and airways and can cause bronchiolitis, pneumonia, coughing, wheezing, or other cold-like symptoms. But for many families, that’s only the beginning. A national survey of parents and healthcare providers found that the disease also leaves an emotional, financial, and social burden. Of the 340 parents whose child caught the virus, more than two-thirds said it landed their child in the hospital. 68% of parents reported the experience affected their mental health while their child was sick. Parents felt afraid, sad, helpless, and frustrated. Many felt guilty they couldn’t do more to prevent their child’s sickness. RSV also dealt excessive financial hardships to black families, who faced medical bills, loss of potential income, childcare costs for siblings, and transportation expenses.
Meanwhile, some parents had to request paid time off, take unpaid leave, or cut back on work. Nearly 20% left their job or were fired as a result. Perhaps that’s why more than two-thirds of surveyed parents described RSV as a financial burden or financial crisis. RSV impacted families’ social balance, too. Over one-third of parents said the experience strained their relationship with their partner. They had to turn to family members and friends to help with childcare, and all the while, siblings struggled to understand what was happening. RSV’s impact is multifaceted. So, how can policymakers help? [They can help by] supporting innovation and ensuring timely and equitable access to care and preventive interventions. Surveyed healthcare providers agreed that immunization and vaccine-like interventions could help minimize the burden of RSV. 82% of parents agreed they would want their child to receive such an intervention with good policy and innovation. Families and their healthcare providers can work together to reduce the burden of RSV.
Susan Hepworth: As you can see there, the burden of RSV goes but the burden extends to the family as well, as represented in those survey results. I want to welcome Dr. Hopkins from NFID, who will give us a presentation about the overview of RSV.
Bob Hopkins: I appreciate you all inviting me to be here. I want to spend just a few minutes discussing RSV disease and epidemiology. To set the stage for those who may not know about the NFID, the National Foundation for Infectious Diseases, a 501(c)(3) organization that was founded in 1973 with the goal of healthier lives for all through effective prevention and treatment of infectious diseases, through education, engagement of the public and other partners in collaboration to improve the health of all.
RSV is a widespread respiratory illness. From the scientific standpoint, it’s an enveloped negative-strand RNA virus from a family known as Pneumoviridae. There are two major subtypes of RSV known as A and B, and there are numerous different phenotypes or genetic groups, but A and B are the two that we need to consider. The symptoms of RSV overlap with other respiratory pathogens like COVID-19, influenza, the common cold, and others. In infants, RSV is the most common cause of bronchiolitis and pneumonia in children aged under one. Those at the highest risk are premature infants, those that have heart and lung disease, and all children, even children born at normal term with no other health issues less than six months of age. Most of those who are infected with RSV have a mild upper respiratory illness or cold, a classic illness that many of us in pediatric practice are used to seeing, or a child that comes in with a cough, then over a day or two develops fever or wheeze and copious nasal drainage area. RSV can cause that, but it can also cause other respiratory symptoms.
Adults, mainly those who are older age and who have chronic health conditions or those who are immunosuppressed, are also at increased risk for severe disease. Our focus is on the neonatal childhood burden, but it’s essential to recognize that there’s also significant disease in older adults.
Did you know that RSV is a common respiratory disease? In most years, RSV circulates in the fall and winter months in the U.S. It often starts earlier in the country’s southeastern part. I’ll show you some of that epidemiologic data in a moment. It’s spread through contact with others and in contact with contaminated surfaces. Unfortunately, the RSV virus can live on hard surfaces for many hours. It’s often spread through coughing, sneezing, kissing, or touching those infected surfaces and then touching your nose, mouth, or eyes. So, as I tried to teach my children and patients, keep your hands away from your face as much as possible.
Almost all children are infected by two years of age, but unfortunately, immunity following RSV infection is not durable. It’s not uncommon to see children and adults who get RSV multiple times a year. This is data from our friends at the Centers for Disease Control showing the seasonality of RSV from the 2018 season through the 2023 season (Table 1). The dark green line here with the high peak over October-November was a 2023 season. We had a very early onset and a severe RSV season in 2021, 2022, and 2023. In the following line that you see down in the panel to the left, you see it was a less severe season, less of a peak. And then, in the other colors, you see different seasons. We’ve seen a change in seasonality patterns, some early and others late; some years are more severe than others.
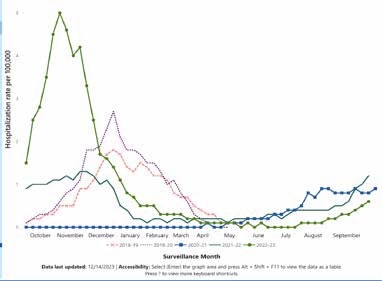
With RSV, we see outbreaks in our population almost every year with slight differences in timing. The incubation period after exposure is typically considered 4 to 6 days, and you can transmit it to others before you develop symptoms. Generally, you can transmit the virus within three to eight days of infection. As previously mentioned, the typical symptoms are runny nose, cough, fever, and sneezing, and in small infants, you might see fussiness, wheezing, decreased appetite, irritability, and reduced feeding. It would be best to be suspicious about RSV when there’s RSV in the community or the time of year.
We expect RSV and its symptoms not to be reliably distinguished from other respiratory viruses, which makes testing very important to distinguish between influenza, COVID-19, and other viruses. PCR or molecular testing is the most accurate way to test for RSV, although antigen tests are also reasonably accurate in children. Recovery from the illness usually takes 1 to 2 weeks. Still, it’s essential to recognize that those persons who are immunocompromised can continue to shed RSV virus, which can infect others for up to a month even after their symptoms are resolved. So, beyond thinking about taking care of yourself, you also need to think about what we can do to prevent transmission of RSV to others. COVID-19 and RSV are uncommon. COVID-19, you commonly see difficulty breathing, a little bit less so with RSV, but that’s still a common symptom that you can have on RSV. For people of all ages who have more severe diseases, fatigue is not typically a common issue with RSV. Fever can occur with any of these illnesses, although less likely in colds, and we think of loss of taste and smell as COVID-19. Sore throat is uncommon with RSV; wheezing is common in RSV patients. So again, RSV illness tends to be most severe in premature infants, infants less than six months of age, and persons with immunocompromised heart and lung diseases.
It is important to remember that over 80% of children who were hospitalized with RSV before two years of age have no risk factors. RSV can affect people regardless of whether they have chronic health conditions or not. RSV is the number one cause of bronchiolitis and pneumonia in children. Every year, approximately 2.1 million outpatient visits a year in children due to RSV illness, with 58,000 to 80,000 hospitalizations a year and, unfortunately, 100 to 300 pediatric deaths in children under five a year. This doesn’t even include the significant additional burden of RSV disease in older adults. So, what’s the treatment for RSV infection? We can suction those copious nasal secretions I mentioned. Oxygen can be provided for those with low oxygen saturation; bronchodilators may help with some of the coughs but have not been shown to change the direction or the duration of illness and nutrition support. It is essential to provide nutrition to help those infected with RSV use their muscles to breathe effectively. There are no currently effective available antiviral medications for us. So that’s one of the reasons that prevention of RSV is so vital. If we can prevent somebody from getting the infection, we don’t have to worry about whether we have effective antivirals.
Regarding the prevention concepts around RSV, hand-washing, surface decontamination, and masks probably have a modest effect on reducing RSV transmission. Still, they are essential, and we should implement these in our daily lives, particularly in the clinical setting. It’s also important to remind people that if you’re sick or your child’s sick, don’t get them out around others or take a chance on transmitting the virus to others. We have selective benefits. Palivizumab is a monoclonal antibody that’s been recommended and approved. The AAP recommendations are to use it for the highest-risk infants. It has to be administered intramuscularly and once a month throughout the season it’s used. We have great potential with our new preventive tools, including honesty, vaccines, and the app, which Dr. Johns will discuss shortly.
That is my brief presentation. I look forward to answering some of your questions in the webinar.
Susan Hepworth: Thank you, Dr. Hopkins. Before I turn it over to Dr. Jones to discuss those prevention tools Dr. Hopkins just spoke about, I want to share the video of one more family who RSV impacted.
Video https://www.infanthealth.org/rsv#videos
Melanie: Life in the Rogers House is very chaotic. My name is Melanie, and this is my husband, Dan. We live in the suburbs of Chicago with our four kids. Our kids are nine, Dylan, six, Reagan, and then we have twins who are three, Austin and Holden. Reagan was four months old, and when she woke up in the morning, I could tell her breathing was not right. Those ten days in the hospital were grueling. They were exhausting.
Dan: The one word I would use to describe the RSV experience is helpless. I never even heard of RSV before. So it’s like, oh, they have RSV, and you’re like, what’s that?
Melanie: Her breathing was not right. She was breathing very deeply, very quickly, and it was really scary. It’s a waiting game. When they finally did tell us that we could go home. I’d be lying if I said I wasn’t panicking, thinking, can I do this at home by myself? It’s very frustrating because there’s nothing we can do to speed up their getting better, and you’re just stuck. As a family of six, we tried to do what we could to keep them from it. We still got it with the twins. For everyone who’s had a child or has dealt with hospitalizations, the bills are all calling at once. They start trickling in; you don’t know when they will stop. You have all the emotions flowing through when it’s happening. You feel helpless because you’re there with your child in the hospital, and then you feel guilty because you’re not at home with the other children. It can be really scary with how contagious it is. You need to trust your instinct with something like this because even though some may say it’s just a cold, these babies cannot handle it. They need supportive care and help. Be bold and call your pediatrician’s emergency line in the middle of the night. If you’re worried about their breathing, don’t be afraid to show up at the E.R. when uncomfortable with your baby’s breathing. This is not something to take lightly. It’s very scary, and you feel very helpless, and this is just something we shouldn’t have to watch our children go through.
Susan Hepworth: The good news is that hopefully, with two newly approved prevention tools available, fewer families will have to experience what the Rogers did, which was three of their four children being hospitalized with RSV. Dr. Jones, I’ll now turn it over to you.
Jefferson Jones: Thanks so much for having me today and for the other presentations. We, as general pediatricians, were undoubtedly excited about this time of being able to prevent severe disease from RSV. So today, I’ll discuss our two new immunization products and CDC recommendations for their use. First, I’ll be going over the efficacy and safety. The two products are nirsevimab and the Pfizer maternal RSV vaccine. Then, the CDC recommendations and clinical guidance for health care facilities. These are assuming a sufficient nirsevimab availability with respect to the shortage of nirsevimab and interim recommendations for healthcare facilities experiencing limited availability. Finally, there are considerations for implementing these RSV immunizations.
First is efficacy and safety. Two products could protect infants in their first RSV season. The maternal vaccine for pregnant people is from Pfizer, and the trade name is Abrysvo. Then, the monoclonal antibody or nirsevimab with the brand name Beyfortus. This is given to the infant after birth. Please note that there is an additional RSV vaccine by GSK with a trade name, Arexvy, which is not approved or recommended for use in pregnant people.
To protect eligible children at increased risk in their second RSV season, the only option is nirsevimab. The efficacy of nirsevimab was initially evaluated through two multi-country trials, including preterm and term infants. Efficacy was assessed 150 days after injection in the trials, and the pooled efficacy from these two trials was 79% in preventing medically attended RSV, lower respiratory tract infection (LRTI), or allergy and then 80.6% in preventing RSV LRTI with hospitalization. Nirsevimab has an acceptable safety profile, and it’s generally well tolerated. The most commonly reported adverse reactions were injection site reactions and rash, which were present in less than 1% of recipients. In trials, the incidence of serious adverse events was not significantly different between the nirsevimab placebo arms.
The efficacy of Pfizer’s maternal RSV vaccine was also evaluated in a multi-country trial, and the vaccine was administered during 24 through 36 weeks gestation. The efficacy was assessed through 180 days of birth, and it was 51.3% in preventing medically attended RSV associate LRTI and 56.8% in preventing hospitalization for RSV-associated LRTI; the side effects tend to be mild or moderate and temporary like those experienced after other vaccinations and the most common local and systemic adverse reactions during the trials were pain at the injection site, headache, muscle pain, nausea, more preterm births, and reports of hypertension during pregnancy, including pre-eclampsia receiving the vaccine group, as well as the placebo group in the clinical trials. However, these differences were not statistically significant, and whether these were related to the vaccine or simply due to chance is unknown. So restricting vaccination to 32 to 36 weeks, as discussed in the recommendations, also reduces any potential risk of preterm birth. The Advisory Committee for Immunization Practice (ACIP) judges that the benefits of maternal RSV immunization at 32 through 36 weeks gestation outweigh any potential risk for preterm birth and hypertensive disorders of pregnancy.
Next, we’ll talk about maternal vaccine recommendations. With seasonal administration, the maternal vaccine is recommended for pregnant people during 32 to 36 weeks of gestation. This means administering from September through January in most continental United States. However, in jurisdictions with seasonality that differs from most of the continental United States, for example, Alaska, and many jurisdictions with tropical climates, a provider should follow state, local, or territorial guidance on timing of administration.
The maternal Pfizer vaccine can be simultaneously administered with other indicated vaccinations. Now, either of the two options, maternal vaccination or the use of nirsevimab in the infant, is recommended to prevent RSV LRTI. However, administration of both products is not needed for most infants. Healthcare providers of pregnant people should provide information on both products and consider patient preferences when determining whether to vaccinate the pregnant patient or not and rely on the administration of nirsevimab to the infant after birth.
Later, I will go into more detail on vaccine counseling and the importance of discussing the potential lack of nirsevimab availability as part of this conversation.
Now, we’ll talk about nirsevimab recommendations. It will first apply to healthcare settings where there’s sufficient supply. In most of the United States, the RSV season has started. Therefore, the administrator and eligible children should begin nirsevimab as soon as it is available. Nirsevimab should continue to be offered to eligible infants and children through March, and it is mainly vital for those born between October 2023 and March 2024. Infants born shortly before the RSV season or in October 2023 through March 2024 should be immunized with nirsevimab within one week of birth, and administration can occur during the birth, hospitalization, or in the outpatient setting. We encourage immunization of infants with prolonged birth hospitalization shortly before or promptly after discharge. For all other infants younger than eight months, nirsevimab should be administered as soon as it is available if the infant is younger than eight months at the time of immunization. Again, this assumes sufficient doses, and I’ll discuss the recommendations if there is a lack of dosing. And because the maternal RSV vaccine is shown to be effective, if the mother was vaccinated 14 or more days before birth year [date of birth], nirsevimab is not needed for most infants.
There are rare circumstances for which nirsevimab can be considered when the mother has received an RSV vaccine 14 or more days before birth. These are when the clinical judgment of a health care provider for the potential incremental benefit of the nirsevimab administration is warranted. Some examples include but are not limited to, infants born to pregnant people who may not mount an adequate immune response to vaccination or have conditions associated with reduced transplacental antibody transfer. So, this could include pregnant people with immuno-compromised conditions. Another example is infants who might have experienced loss of maternal antibodies. These could consist of infants who undergo cardiopulmonary bypass or ECMO and then infants with substantially increased risk for severe RSV disease, such as hemodynamically significant congenital heart disease for infants that have experienced ICU admission and are requiring oxygen at the time of discharge back at home. Nirsevimab should be administered if the age is 8 to 19 months at the time of the immunization and the child is at increased risk for severe disease, including children with chronic lung disease of prematurity, children with severe cystic fibrosis, children with severe immunocompromised and American Indian or Alaska Native children.
Next, I’d like to discuss CDC interim recommendations for settings lacking nirsevimab availability. For the current season, the manufacturers reported a limited supply of nirsevimab, particularly the 100-milligram prefilled doses. Based on manufacturing capacity and currently available stock, there needs to be more 100 mg prefilled syringes and/or said MAB to protect all eligible infants weighing five kilograms or more during this current RSV season. Additionally, the supply of the 50 mg prefilled syringes may be limited. On October 23rd, 2023, the CDC released a health advisory describing interim recommendations to provide options for clinicians to protect infants from RSV. In this context of a limited nirsevimab supply, the recommendations for the 50-milligram doses remain unchanged. But to help preserve the 50-milligram doses, providers should encourage pregnant people to receive Pfizer’s maternal RSV vaccine during 32 to 36 weeks gestation to prevent RSV-associated LRTI.
In the conversation between providers and pregnant people, stress that there’s a limited nirsevimab availability when they’re deciding whether or not to receive the RSV vaccination during pregnancy. Now, in healthcare settings with limited 100-milligram doses, providers should prioritize infants at the highest risk of severe RSV disease for receipt of 100-milligram doses. These include infants that are younger than age six months, American Indian or Alaskan native infants aged less than eight months, and infants under age six to eight months that have conditions that place them at high risk of severe RSV disease. These include those that were born prematurely at less than 29 weeks gestation, chronic lung disease, hemodynamically significant congenital heart disease, severe immunocompromised or cystic fibrosis, and neuromuscular disease or congenital pulmonary abnormalities that impair the ability to clear secretions. Additionally, 50-milligram doses should be reserved only for infants weighing less than five kilograms, meaning providers should avoid using two 50-milligram doses instead of a 100-milligram dose for infants weighing five kilograms and more. As mentioned, that’s because the youngest infants are at the highest risk for severe disease. So prioritizing doses for them is crucial, and providers should follow AAP recommendations for eligible infants when the appropriate dose of nirsevimab is unavailable. In addition, these are for healthcare facilities with limited availability of nirsevimab. Providers should suspend the use of their nirsevimab for children aged 8 to 19 months, with these children receiving nirsevimab per AAP recommendations. Providers should continue offering nirsevimab to American Indian or Alaska Native children aged eight through 19 months, particularly those who live in remote regions where transporting children with severe RSV who need an escalation of medical care may be challenging. These communities have known high rates of severe RSV among this age group.
Lastly, I’d like to review some considerations regarding the cost of implementing these RSV immunizations. The maternal RSV vaccine is $295 per dose. Medicaid should cover the cost without cost-sharing. The VFC program is available for persons under 19 years, and most private insurance plans are required to cover the maternal RSV vaccine but have approximately one year to do so. For nirsevimab, the price is $495 per dose, which is the private sector cost, and payment flexibilities exist this season. For example, providers have 150 days for payments when ordering directly from the manufacturer. For insurance coverage, nirsevimab is covered under the Vaccines for Children program, and most private insurance plans require coverage of nirsevimab, but also one year to do so.
The storage handling administration for these two products, for the maternal RSV vaccine, is supplied as a three-component kit and requires reconstitution. It has the lyophilized antigen vial, sterile water diluent syringe, and vial adapter. Before reconstitution, it should be stored at refrigerated temperatures and not frozen. After reconstitution, it should be stored at room temperature and used within 4 hours.
The maternal RSV vaccine is given similarly to other vaccines as an intramuscular injection in the deltoid muscle or thigh. The dosage is 0.5 milliliters. Nirsevimab is supplied in two doses, 0.5 milliliter or 60 milligrams, in a prefilled syringe with a purple plunger rod, [and] one milliliter or 100 milligrams prefilled syringe dose in a light blue plunger rod. Nirsevimab should be stored at a refrigerated temperature and used within 8 hours of being removed from the refrigerator, and it should not be frozen, shaken, and protected from light.
Nirsevimab is administered similarly to traditional vaccines, such as an intramuscular injection in the Vastus lateralis muscle of the anterolateral thigh. Nirsevimab is dose-dependent on weight. For those that are less than five kg, 50-milligram doses will be given, and those in their first RSV season, then for those who are five kg and greater, receive one 100-milligram dose. Children at increased risk of severe disease entering their second RSV season and will be 8 to 19 months of age receive two 100-milligram doses to make up a 200-milligram dose. The maternal RSV vaccine and nirsevimab can be administered with other recommended vaccinations, though there are some considerations for counseling patients on the RSV vaccine. As mentioned, either the maternal RSV vaccine or nirsevimab is recommended for all infants.
Administration of both products is only needed for some infants. Providers of pregnant people should discuss both products and consider the relative advantages and disadvantages of each product, which I’ll review next. Patient preferences and the availability of nirsevimab are fundamental. Thus, prenatal providers who do not offer the maternal RSV vaccine should refer patients elsewhere for vaccination. We ask that providers proactively provide a prescription if state law requires vaccination in a pharmacy. This is important because Nirsevimab has limited availability in some areas.
Encouraging maternal vaccination, particularly this season, is essential. Some of the relative advantages that could be discussed are that the maternal RSV vaccine has the advantage of providing protection immediately after birth, which is when infants are at the highest risk for severe disease. The maternal RSV vaccine also might be more resistant to potential mutations in the F protein. RSV does not typically mutate rapidly, but this could occur. Some relative disadvantages are potentially reduced protection in some situations. For example, if the pregnant person is immunocompromised or the infant is born soon after vaccination, then there’s also the potential risk for preterm birth or hypertensive disorders of pregnancy.
The advantages of nirsevimab include protection from nirsevimab, which may wane more slowly than the maternal RSV vaccine; nirsevimab is a direct receipt of antibodies. It does not rely on placental transfer from the pregnant person to the fetus. Additionally, there’s no risk for adverse pregnancy outcomes. There are some disadvantages, and, importantly, as mentioned several times, there’s limited availability during the 2023 to 2024 RSV season. Further, nirsevimab also requires an infant injection.
A vaccine information statement for the maternal RSV vaccine and an immunization information statement for the nirsevimab are available. It is critically important to document receipt of the maternal RSV vaccine as most infants of vaccinated mothers are not recommended to receive nirsevimab. This can include recording it in an immunization information system, the electronic health record, and written or printed documentation needed for the parent to bring to the birth hospital and pediatrician; this may depend on state policy or laws.
In summary, RSV can cause serious illness in infants and children, and this includes hospitalization and potentially death. To protect eligible infants in the first season, the maternal vaccine or nirsevimab is recommended to prevent RSV and LRTI in infants. Still, administration of both products is optional for most infants. The maternal vaccine from Pfizer and nirsevimab, to protect eligible infants in their second season, nirsevimab is recommended regardless of maternal RSV vaccination. Then, a similar simultaneous administration of nirsevimab with age-appropriate vaccines is recommended. Maternal vaccine is recommended for pregnant people during 30 to 36 weeks gestation, from September through January. It’s recommended in most continental United States, but in certain jurisdictions, the seasonality differs; providers should follow state, local, or territorial guidance on the timing in infants younger than eight months. Infants born during or entering their first RSV season are recommended to receive one dose of nirsevimab if the mother did not receive the RSV vaccine during pregnancy, if the mother’s RSV vaccination status is unknown, and the infant was born within 14 days of maternal RSV vaccination. Finally, children aged eight through 19 months who are at an increased risk of severe disease while entering their second RSV season are recommended to receive one dose of nirsevimab when available.
Susan Hepworth: Thank you so much, Dr. Jones, for all that information. I’ll turn it over to Dr. Kieren Crowley to discuss patient and provider education resources.
Kieran Crowley: When we think about educating our patients from a provider perspective, it’s vital that we consider many factors, especially when discussing vaccinations. It is recommended that preventative health discussions start early and occur throughout the pregnancy, addressing maternal immunization and the infant option. In the prevention of RSV, this anticipatory guidance helps to provide several opportunities for patients and providers to discuss the disease-specific condition we’re discussing, in this case, RSV, and the health implications it has on the infant. It’s an opportunity to provide preventive health treatment options, including maternal immunization, Pfizer, or the infant preventative treatment, nirsevimab, and how each works to prevent the infant from becoming infected and potential side effects.
It’s also essential when discussing vaccinations throughout the pregnancy before the seasonality of the recommendation timeline so that the patient and family can make an informed decision and share their preferences regarding their fears and concerns with their provider. Part of the education should also include if there are specific reasons why we would avoid or delay the vaccination; in this case, any vaccination avoidance would consist of any history of severe allergic reactions. For the RSV vaccine or treatment options, a delay in administering at a set visit would occur if that person presented with severe signs and symptoms of illness, regardless of whether they might have a fever. I know Dr. Hopkins and Dr. Jones already addressed some of the seasonality issues that we would see in some parts of the United States with a different vaccination schedule. Suppose you’re a provider within those states, such as Alaska, Florida, and the U.S. territories. In that case, it’s important to discuss those differences regarding the timing of the vaccination with the individual patient so that they’re aware of those differences because they may see different messages and websites. In the content that we give, we give them for education. Being proactive in that discussion is essential, and then discussing the simultaneous administration with our other maternal vaccines is important because it is approved for concurrent administration with the flu, Tdap, and COVID-19 vaccinations.
When we talk about patient resources, individuals learn differently, and using varying methods helps to increase comprehension. It makes for a better-informed decision from the patient’s perspective. Utilizing varied methods is very important, as you see in this webinar. The use of videos, infographics, and written take-homes is essential. Having verbal and repetitive conversations throughout the pregnancy is helpful so they can identify questions that might need to be answered, and they can share their concerns and fears, which can be addressed factually by the providers. Sharing reputable websites that provide information at a patient level is essential. Everyone uses Google efficiently, but it may guide them to inappropriate websites. We want to help educate our patients on determining which websites are best for them and adults at a level where they can understand the materials they’re reading; if they have any questions about those, bring them back to the provider.
There are a couple of patient resources that I can mention; one is the CDC, which has several infographics that you saw in Dr. Hopkins’s and Dr. Jones’s presentations. These infographics can be used for provider and patient perspectives on different vaccines. We also have various languages to help provide language-proficient infographics to our populations. Another is AWHONN, which has “Healthy Mom and Baby” information. This consumer-facing and patient education platform offers various information on pregnancy, family, childbearing, and childraising issues, including maternal immunizations and the RSV vaccine. Other information sources are ACIP and ACOG. The CDC also has a convenient vaccine schedule that all patients will hopefully follow regarding infant to adult immunization schedules, as presented by Dr. Jones. The fact sheets and the vaccine information statements are crucial and can be used throughout the different visits to help inform the patient of those recommendations.
Regarding providers and their resources, utilizing a professional or a national organization such as the FDA, CDC, and ACIP on the approval and recommendation protocols seen with different vaccinations is extremely important to acknowledge. Each provider professional organization, whether at different provider levels or in specialties, also has statements and/or provides guidance practice advisories on those websites that help guide the professional provider with recommendations to the populations they serve. An example is ACOG for obstetrics and gynecology. The nurse, midwives, pediatrics, and family practice have similar resources. One resource that I find very, very helpful, and that came to light during the COVID-19 pandemic, is the CDC State of Vaccine Confidence and site reports, which provide a thematic analysis of potential impacts on the vaccine, confidence of the public and the demands that we might see out there across the United States.
In addition, thematic analysis provides talking points and strategies to overcome those themes for us to prepare for conversations that might arise during our visits with our patients. They also offer websites that provide fliers and posters for offices to use, which is very helpful. While patients sit in waiting rooms and exam rooms, that helps them think of questions. The websites also have toolkits that help provide social media dissemination opportunities for other providers.
Key take homes are it is essential providers make sure that they are talking early in the pregnancy and frequently throughout the pregnancy, addressing maternal and or newborn treatment prevention options, and providing people information on those options so that in a non-judgmental way, each individual can have their process for decision making. Having that be a shared decision helps reach the goal of the best preference for that person and their infant and prevents RSV. Many of us already know that there’s a plethora of research on the adoption of vaccines that is consistent in finding that patient adoption stems from the provider’s recommendation. Research on when and how a provider might recommend and administer vaccines is highly associated with a national professional organization recommendation. For the RSV vaccine, we have that from the FDA, CDC, and professional organizations. Now, it’s up to the healthcare team to educate the patients, recommend treatment, and work with patients to understand their preferences for preventing RSV. I can take any questions at the end as well. Thank you.
Susan Hepworth: Thank you, Dr. Crowley. We’ll invite Dr. Hopkins and Dr. Jones to also come on screen. We have about 8 minutes, and we have received a lot of questions that have come in. We’re just going to dive right in. I will allow any of you who want to answer to go ahead and do so. Let me start with: “Why is the maternal vaccine only recommended in September through January versus through March, like nirsevimab?” Dr. Jones, that might be for you.
Jefferson Jones: The most significant difference between nirsevimab and the maternal vaccine, when it’s given, is when protection begins. By the end of March, the RSV season is not an on-and-off; it goes up and then slowly peters off over March and April during pre-pandemic months and in nirsevimab as soon as you give the monoclonal antibody, almost immediately after, protection starts. For the maternal vaccine, you’re giving it 30 to 36 weeks, so, for many, it will be up to eight weeks before that infant is born. For infants born after the RSV season or just towards the end of last season, ACIP felt it wasn’t a good use of resources.
Susan Hepworth: As a subsequent question: “Shouldn’t nirsevimab be administered to a baby that has tested RSV positive this season?”
Bob Hopkins: I’d be happy to take that one, Suzanne. Remember that Nirsevimab is trying to prevent RSV infection; one RSV infection does not protect you from additional RSV infections. We also discussed that immunity following RSV infection is minimal. If you have an infant that has an early RSV infection, if they’re still in that risk window period, then you could use nirsevimab for prevention of further severe infections.
Jefferson Jones: That’s right. The AAP has mentioned that for those that have shortages of nirsevimab, they say you could consider it a non-priority if you don’t have enough availability for those that have already been infected, you may not need to give it that the benefit may not be as much as people who haven’t been previously infected. But Dr. Hopkins is correct.
Susan Hepworth: Next question: “It was mentioned that at-risk infants entering their second season should get two 100-milligram doses of nirsevimab. Are they given at the same time?”
Jefferson Jones: That’s a short one; yes, they are standard. Two immunizations in space, at least one inch apart.
Susan Hepworth: Great. This is a question I have been asked a couple of different ways here: “Do we know how long the maternal vaccination antibodies will last versus nirsevimab?”
Jefferson Jones: This is a bit more complicated. There hasn’t been a lot released on the maternal vaccine yet. They are in phase two and have presented some preliminary data and presentations. What we do know is that the efficacy was there. Primary outcomes were measured at 0 to 90 days and 0 to 180 days. We only showed the 0 to 180-day outcomes; for time’s sake, we kept it shorter. However, the 0 to 90-day efficacy is higher for the maternal vaccine. So, some waning may be from 90 to 180 days for efficacy. There may be protection that lasts beyond 180 days; there are probably antibodies, but we need to know how many antibodies you need to protect from RSV. For now, there’s confidence that there’s protection until 180 days after birth. We don’t know for sure after that. Based on other vaccines that are given during pregnancy, there’s pretty limited, if any, protection offered much later than that.
On nirsevimab, antibody data has been published that shows there is waning over time about a year after nirsevimab is given. It’s higher for those who have been given nirsevimab compared to those who were infected during their trials, which is encouraging, but we only know efficacy. It does protect infants for 150 days after administration. Of note is that nirsevimab has been engineered and changed. It lasts longer in the body, the half-life is longer, and compared to antibodies, it persists longer. But how exactly that will lead to potentially prolonged protection beyond 150 days will require further studies, which we’re currently working on based on ongoing studies conducted by CDC and other partners.
Bob Hopkins: I agree with what you said. Many will continue asking about the comparative effectiveness of nirsevimab as opposed to maternal immunization. And until we truly have somebody look at a comparative effectiveness trial and we look at the real-world experience with these products, we will continue to ask these questions. As a practicing Med/Peds doctor, the critical point for me is that we wanted to get as many of these pregnant women vaccinated as possible to pass those antibodies onto their infants. In any of the infants born to women who were not vaccinated, we want to get as many of them protected with nirsevimab in their first season at high risk and in their second seasons to reduce the burden of the number of these kids ending up in the hospital. Unfortunately, some have severe outcomes, including death for three.
Susan Hepworth: Well, we’re out of time and have answered many of the questions that have come in. I have received a lot of questions about whether these presentations will be made available and whether we can get a recording. The Coalition will send a follow-up email with more information to everybody who attended today’s webinar. I want to thank Dr. Crowley, Dr. Hopkins, and Dr. Jones for sharing this essential information with us today, and I appreciate everybody joining. Thank you, and have a great day.
Disclosures: The authors have no relevant disclosures.
Corresponding Author

Susan Hepworth
Director
National Coalition for Infant Health 2020 K Street NW
Suite 505
Washington, DC 20006
Email: info@infanthealth.org

Bob Hopkins, Jr., MD,
National Foundation for Infectious Diseases

Jefferson Jones, MD, MPH, FAAP
Centers for Disease Control and Prevention

Karen Crowley, DNP, APRN-BC, WHNP
Association of Women’s Health, Obstetric and Neonatal Nurses
